JIAOZUO ZHONGWEI SPECIAL PRODUCTS PHARMACEUTICAL CO.,LTD


| Availability: | |
|---|---|
Povidone Iodine (PVP-I) is a stable chemical complex of polyvinylpyrrolidone (PVP) and elemental iodine, appearing as a yellowish-brown to reddish-brown amorphous powder.
It is highly water-soluble, allowing for easy formulation into solutions, ointments, and other dosage forms. PVP-I is widely recognized for its broad-spectrum antimicrobial activity, effectively killing bacteria, viruses, fungi, and protozoa.
It is valued for its low toxicity, excellent skin compatibility, and sustained release of iodine, providing effective disinfection while minimizing irritation.
Structure formula:
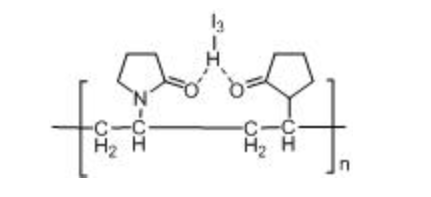
Appearance: Yellowish-brown or reddish-brown amorphous powder
Application: OTC disinfectant, surgical and medical equipment disinfection
Broad-Spectrum Antimicrobial Activity: Effective against bacteria (including resistant strains), viruses, fungi, and protozoa.
Sustained Release of Iodine: Provides prolonged antimicrobial action while reducing toxicity and staining associated with iodine solutions.
Excellent Solubility: Easily formulated into various pharmaceutical dosage forms, including topical solutions, ointments, and scrubs.
Low Irritation and Good Tolerance: Suitable for use on skin and mucous membranes with minimal irritation.
Stable Complex: Maintains efficacy while ensuring low levels of free iodine release to control odor and reduce irritation.
Povidone Iodine | |
Loss on Drying (%) | ≤8.0 |
Residue on Ignition (%) | ≤0.1 |
Iodide Ion (%) | ≤6.6 |
Heavy Metals(ppm) | ≤20 |
Available Iodine (%) | 9.0-12.0 |
Nitrogen (%) | 9.5-11.5 |
All products comply with Ph. Eur, USP/NF, JP,BP certificate of analysis.
Standard Packaging: 25 kg plastic drum or fiber drum, inner lined with PE bags.
Custom Packaging: Available according to customer requirements.
Storage Conditions: Store in tightly sealed containers in a cool, dry place, protected from light and moisture to maintain product stability.
A: PVP-I slowly releases free iodine when in contact with the skin or mucous membranes, which penetrates microbial cell walls and inactivates proteins and enzymes, effectively killing bacteria, viruses, fungi, and protozoa.
A: Yes, Povidone Iodine is highly water-soluble, making it easy to formulate into various dosage forms, including solutions, scrubs, ointments, and gels.
A: The available iodine content ranges from 9.0% to 12.0%, ensuring effective antimicrobial activity while maintaining stability.
A: A Loss on Drying of ≤8.0% ensures minimal weight loss during storage and processing, maintaining consistent concentration and stability in formulations.
A: It should be stored in tightly sealed containers, in a cool, dry place, away from direct sunlight and moisture, to preserve its stability and activity.
Povidone Iodine (PVP-I) is a stable chemical complex of polyvinylpyrrolidone (PVP) and elemental iodine, appearing as a yellowish-brown to reddish-brown amorphous powder.
It is highly water-soluble, allowing for easy formulation into solutions, ointments, and other dosage forms. PVP-I is widely recognized for its broad-spectrum antimicrobial activity, effectively killing bacteria, viruses, fungi, and protozoa.
It is valued for its low toxicity, excellent skin compatibility, and sustained release of iodine, providing effective disinfection while minimizing irritation.
Structure formula:

Appearance: Yellowish-brown or reddish-brown amorphous powder
Application: OTC disinfectant, surgical and medical equipment disinfection
Broad-Spectrum Antimicrobial Activity: Effective against bacteria (including resistant strains), viruses, fungi, and protozoa.
Sustained Release of Iodine: Provides prolonged antimicrobial action while reducing toxicity and staining associated with iodine solutions.
Excellent Solubility: Easily formulated into various pharmaceutical dosage forms, including topical solutions, ointments, and scrubs.
Low Irritation and Good Tolerance: Suitable for use on skin and mucous membranes with minimal irritation.
Stable Complex: Maintains efficacy while ensuring low levels of free iodine release to control odor and reduce irritation.
Povidone Iodine | |
Loss on Drying (%) | ≤8.0 |
Residue on Ignition (%) | ≤0.1 |
Iodide Ion (%) | ≤6.6 |
Heavy Metals(ppm) | ≤20 |
Available Iodine (%) | 9.0-12.0 |
Nitrogen (%) | 9.5-11.5 |
All products comply with Ph. Eur, USP/NF, JP,BP certificate of analysis.
Standard Packaging: 25 kg plastic drum or fiber drum, inner lined with PE bags.
Custom Packaging: Available according to customer requirements.
Storage Conditions: Store in tightly sealed containers in a cool, dry place, protected from light and moisture to maintain product stability.
A: PVP-I slowly releases free iodine when in contact with the skin or mucous membranes, which penetrates microbial cell walls and inactivates proteins and enzymes, effectively killing bacteria, viruses, fungi, and protozoa.
A: Yes, Povidone Iodine is highly water-soluble, making it easy to formulate into various dosage forms, including solutions, scrubs, ointments, and gels.
A: The available iodine content ranges from 9.0% to 12.0%, ensuring effective antimicrobial activity while maintaining stability.
A: A Loss on Drying of ≤8.0% ensures minimal weight loss during storage and processing, maintaining consistent concentration and stability in formulations.
A: It should be stored in tightly sealed containers, in a cool, dry place, away from direct sunlight and moisture, to preserve its stability and activity.